#Industry News
Enhancing Battery Performance: Utilizing FPI ICP-OES to Accurately Assess Metal Impurities in Electrolytes
ICP-OES Test Report
The electrolyte is crucial for lithium-ion batteries, affecting their temperature, energy capacity, cycle efficiency, and safety. Lithium hexafluorophosphate (LiPF6) is a core material used in electrolyte production. However, LiPF6 electrolyte may contain impurities like hydrogen fluoride, water, and metal ions that can negatively impact battery performance. Excessive metal impurity ions can decrease the battery's reversible capacity and prevent proper electrode passivation, potentially leading to battery damage. Therefore, strict limits are set for metal element content in LiPF6 electrolyte.
The electrolyte is crucial for lithium-ion batteries, affecting their temperature, energy capacity, cycle efficiency, and safety. Lithium hexafluorophosphate (LiPF6) is a core material used in electrolyte production. However, LiPF6 electrolyte may contain impurities like hydrogen fluoride, water, and metal ions that can negatively impact battery performance. Excessive metal impurity ions can decrease the battery's reversible capacity and prevent proper electrode passivation, potentially leading to battery damage. Therefore, strict limits are set for metal element content in LiPF6 electrolyte.
Experimental part
Instrument ---- Inductively Coupled Plasma Spectrometer
Model: ICP-OES EXPEC-6500
Configuration: EXPEC 6500D Organic Sampling System
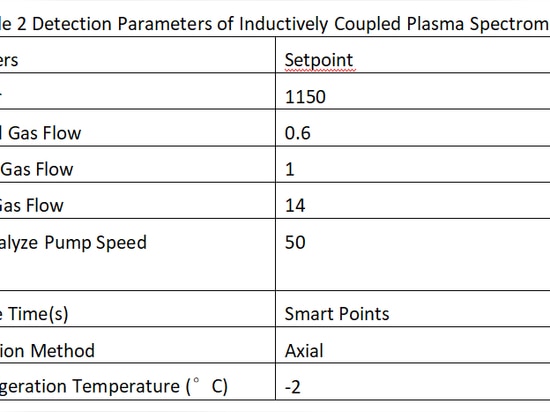
Parameters Setpoint
RF Power 1150
Atomized Gas Flow 0.6
Auxiliary Gas Flow 1
Cooling Gas Flow 14
Flush/Analyze Pump Speed
(rpm) 50
Response Time(s) Smart Points
Observation Method Axial
TEC Refrigeration Temperature (°C) -2
(YOU CAN KNOW MORE IN IMAGE)
Reagents and Standards
Reagents: Electronic grade anhydrous ethanol
Purified water: 18.2 MΩ·cm deionized water
Standard solutions: Al, Ca, Co, Cr, Cu, Fe, Hg, Mg, Mo, Ni, Na, Pb, S, Zn single-element standard solutions, 1000 ug/mL, provided by the National Nonferrous Metals Research Institute.
Sample Preparation
Weigh 2.0g of lithium hexafluorophosphate electrolyte and dilute it with 20% ethanol to a total weight of 10.0g.
Standard Curve and Detection Limit
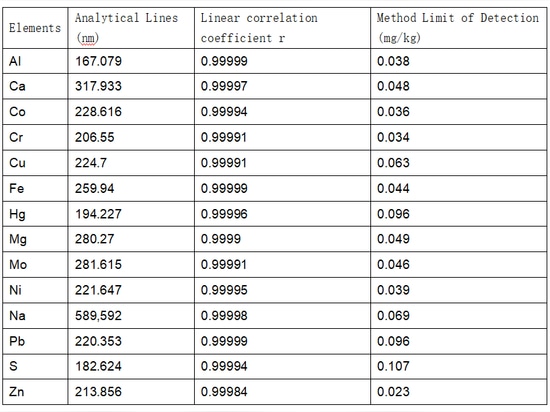
Select appropriate analytical spectral lines for the target elements and plot the standard curve. The test results showed a linear correlation coefficient greater than 0.9990 for the target elements. The concentration corresponding to three times the standard deviation of the measured values from the blank samples, obtained by continuous analysis of 11 replicates, is considered the detection limit of the instrument. The linear correlation coefficient, analytical spectral lines, and detection limits for each target element are shown in Table 3(Pls check related image).
Precision Testing Method
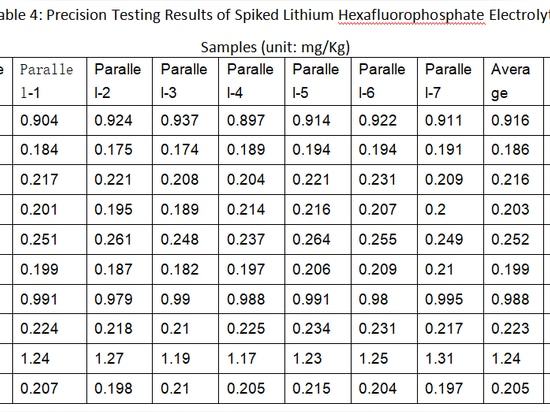
Seven replicate samples of lithium hexafluorophosphate electrolyte, after being spiked with standards, were subjected to secondary testing. The results indicated that the relative standard deviations (RSD) for all elements were less than 5.0%. This demonstrates the excellent precision of the method. The precision testing results for each element in the spiked lithium hexafluorophosphate electrolyte samples are presented in Table 4(Pls check related image).
Actual Sample Spike Recovery Testing
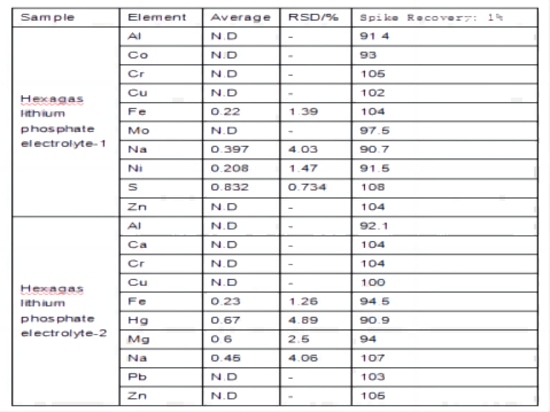
Spike recovery testing was conducted on two different samples of lithium hexafluorophosphate electrolyte. Each sample was spiked with appropriate concentrations based on the element content, as shown in Table 6(Pls check related image). The spike recovery rates for each sample ranged from 90.7% to 108%.
Conclusion
In this experiment, a method was established to determine the content of 14 elements (lead, iron, copper, zinc, chromium, aluminum, sodium, calcium, magnesium, mercury, sulfur, cobalt, nickel, and molybdenum) in lithium hexafluorophosphate electrolyte, which was diluted with 20% ethanol and analyzed using ICP-OES. The experimental results showed that the linear correlation coefficients of the established calibration curves were all greater than 0.9990. The precision testing of the target elements in the actual samples showed relative standard deviations (RSD) below 5.0%. Additionally, the spike recovery rates for the target elements in the actual samples ranged from 90% to 108%. The detection limits for the elements ranged from 0.023 to 0.107 mg/kg. These results indicate that the precision and accuracy of the sample testing were satisfactory, and this method can be applied to determine the content of iron, sodium, magnesium, mercury, sulfur, nickel, molybdenum, and other elements in lithium hexafluorophosphate electrolyte samples.